The coronavirus vaccine is being rolled out across the EU but there have been delays.
image copyrightReuters
The European Union (EU) has said it could block exports of the Oxford-AstraZeneca jab to the UK, amid claims current arrangements are slowing down its own vaccine rollout.
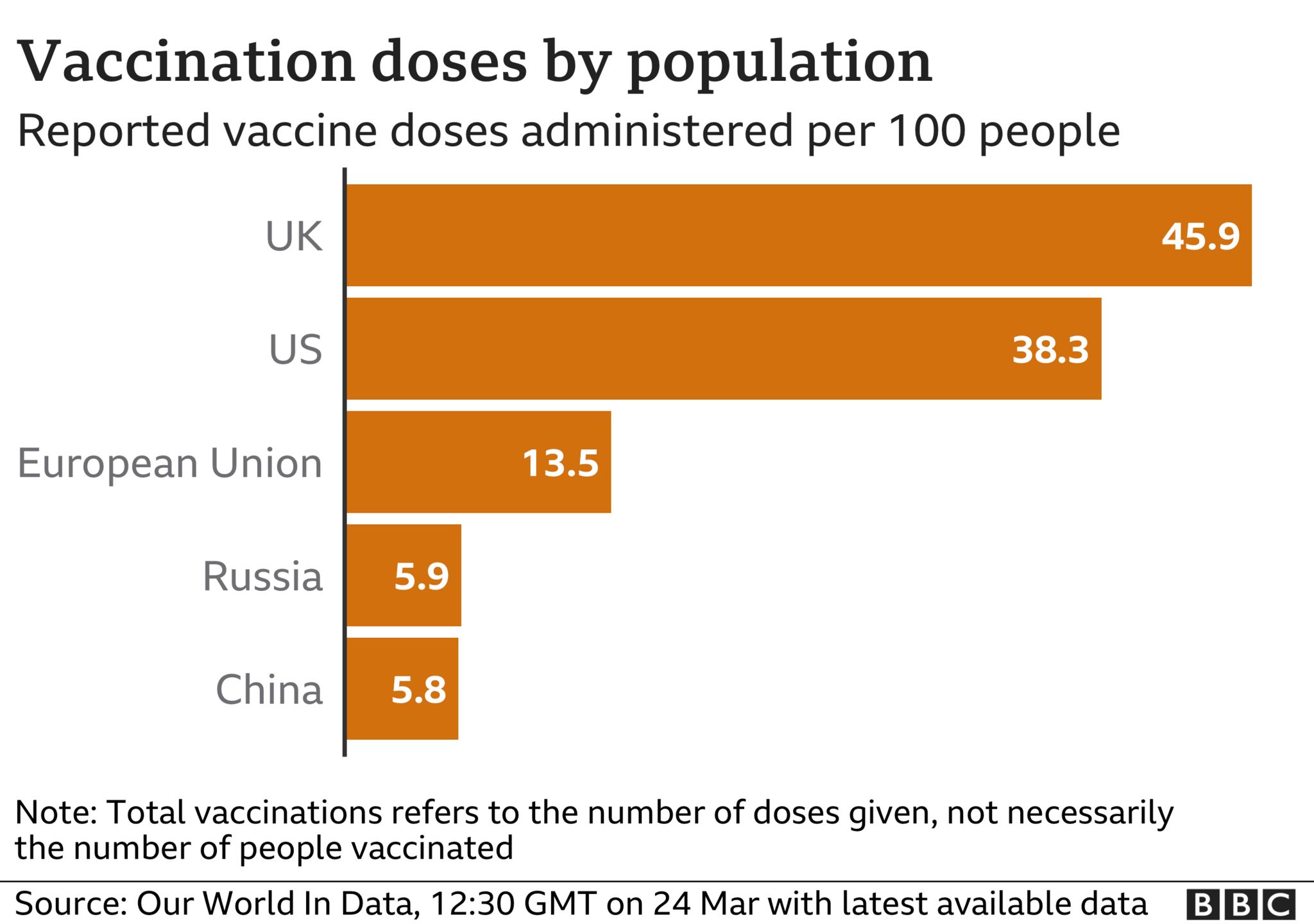
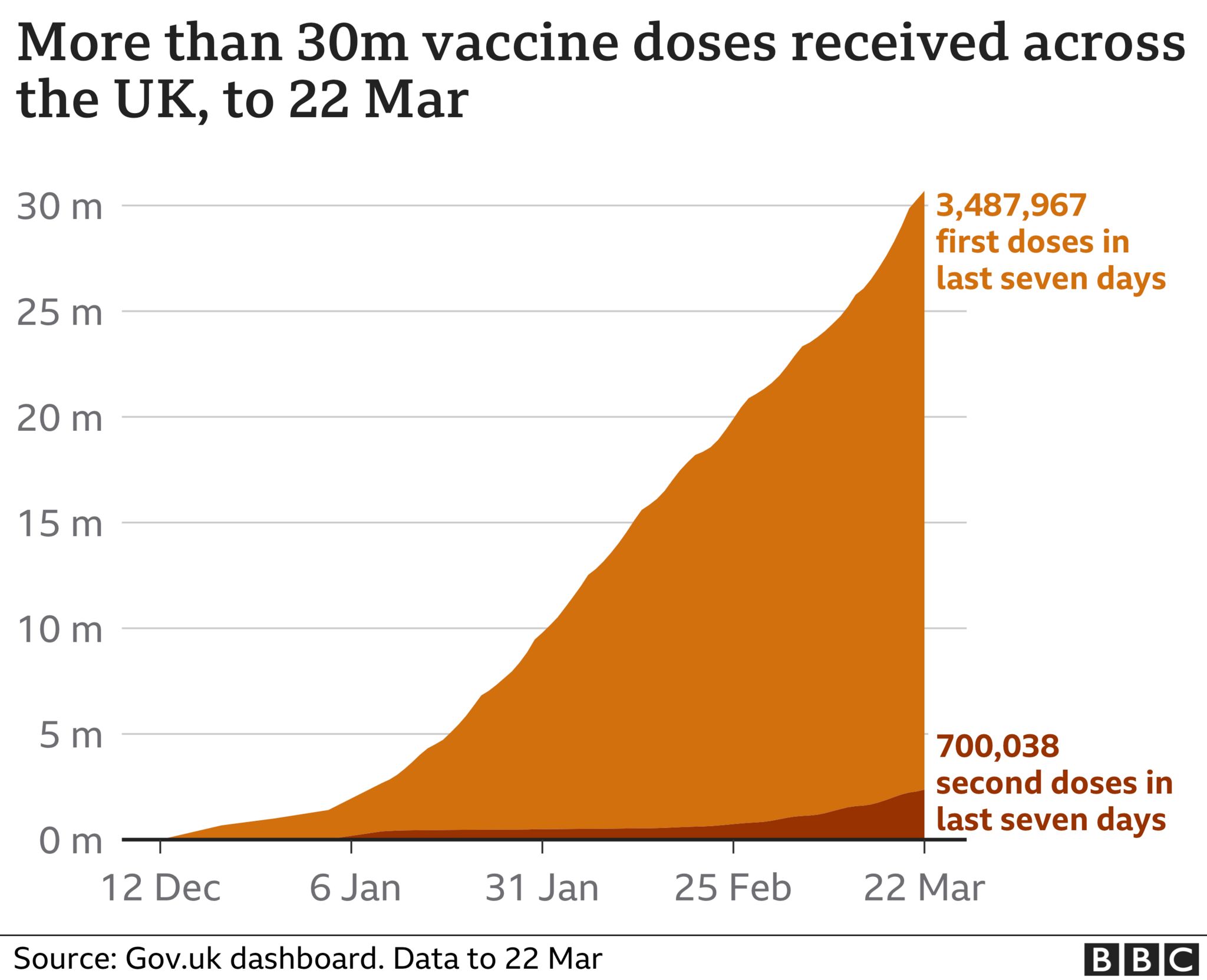
The EU has been criticised for the pace of its vaccination programme – only 14% of its population have received the jab, compared with 45% in the UK.
The EU is concerned that the UK has had an unfair advantage in contracts it signed with vaccine manufacturers, some of whom are based within the EU.
The latest point of contention is over AstraZeneca doses being manufactured in the Netherlands, with an EU official quoted as saying they should be distributed among member states, and not sent to Britain.
On 24 March, the European Commission said that the EU had exported10.9 million jabs to the UK, but that the UK had yet to export any in return.
On Thursday, the EU leaders will consider measures which could stop exports of vaccines to the UK.
Under the proposals, the EU would base its decision on whether to export vaccines to another country on how many jabs or components that country itself was exporting and on its infection and vaccination rates.
In June 2020, all 27 member states joined up to a scheme giving the EU central responsibility for buying vaccines.
However, the EU was slower than the UK to negotiate a contract with AstraZeneca, which caused supply problems.
It also signed deals with Pfizer-BioNTech and Moderna, which ran into early problems with production and distribution.
In February, Ms von der Leyen acknowledged the EU’s vaccine failures, saying: “We were late to authorise. We were too optimistic when it came to massive production and perhaps too confident that what we ordered would actually be delivered on time.”
The EU has reached agreements to buy three other vaccines, once they pass clinical trials – Sanofi-GSK, Johnson & Johnson and CureVac.
In January, the European Medicines Agency (EMA) approved the use of the Oxford-AstraZeneca vaccine for all age groups, but a number of EU countries initially refused to recommend its use for people over 65.
France and Germany eventually revised their stance and approved the vaccine for people aged 65-74 at the beginning of March.
However, they were among 13 European countries who paused the AstraZeneca rollout again in March, after reports that a small number of people developed blood clots after receiving the jab.
They restarted it after the EMA said there was no evidence that the vaccine caused the clots. However, the French authorities say only people aged 55 and over should get the Oxford-AstraZeneca jab.
The headlines surrounding AstraZeneca have led to a drop in confidence in the jab in the EU. The polling company YouGov reports that only a third of Germans and 23% of French respondents now consider it safe.
Member states are allowed to strike separate deals with vaccine makers which have not signed agreements with the EU.
Hungary and Slovakia has bought doses of Russia’s Sputnik vaccine, and the Czech Republic is said to be considering buying it too.
Austria and Denmark have announced they are joining forces with Israel to produce second-generation vaccines against mutations of the coronavirus.
Under the terms of the EU scheme, member states are not supposed to strike deals with any vaccine manufacturer with whom the EU already has an agreement.
However, the German government signed its own side-deal with Pfizer for 30 million extra doses in September.
In January, the European Commission refused to say whether this had broken the terms of the EU scheme.
The UK approved the Pfizer vaccine in November, nearly three weeks before the EU regulators.
The government claimed that being outside the EU allowed it to be more nimble in this area.
However, the UK’s approval of the jab would have been permitted anyway under EU law – a point made by the head of the UK medicines regulator.
The UK could have joined the EU vaccine scheme last year while it was still in a transition phase with the EU, but it chose not to.
If it had, the UK might not have been able to do as many deals with vaccine companies.

